Self-assembly of human neural 3D cell cultures using Nanomed3D PASAP1 hydrogel and cellQART® Cell Culture Inserts
Experiments have been performed by Nanomed3D’s R&D (nanomed3d.com) team.
Written by: Karina Cuanalo-Contreras, PhD
Abstract
The study of the human brain has been limited by the availability of relevant models. Recent advances in 3D cell culture and cellular differentiation, offer promising alternatives to study normal and pathological conditions of the brain in a human relevant context. 3D cell culture allows cells to grow and interact in a platform that simulates in vivo like conditions. Many 3D cell culture techniques involve the use of scaffolds to support cell growth. However, many commercially available scaffolds are of animal origin and difficult to manipulate, leading to variability and ethical concerns. In this regard, synthetic hydrogels offer an excellent, defined and reliable alternative. Here, we describe the 3D self-assembly, and differentiation of human neural stem cells embedded in PASAP1 – a 100% animal-free synthetic matrix from Nanomed3D – grown on cellQART® Cell Culture Inserts made by SABEU.
Introduction
Nowadays, there is a huge interest to develop physiological relevant human in vitro CNS models to study brain development, learning, plasticity and neurological disorders such as Parkinson’s or Alzheimer’s disease.
Whereas animal and 2D cellular models have been useful to partially understand brain physiology and pathology, they are incomplete, and don’t recapitulate essential brain aspects 1. Therefore, there is an urgent need to generate efficient models.
In contrast to 2D cell cultures, 3D cell culture technologies resemble in vivo environments and the physiological complexity of the organ or tissue of origin 2. 3D cell cultures represent highly relevant in vitro models where human cells can be incorporated. Currently, it is possible to derive stem cells from biopsies of patients (iPSCs), differentiate them into distinct nerve cells and grow them into three-dimensional brain-like structures 3.
Most 3D cell culture techniques incorporate a 3D scaffold where cells are embedded. The scaffold provides in vivo like support for cells. Scaffolds of biological origin have been widely used to generate 3D cell culture models; however, their use impose experimental disadvantages 4. In addition, biological scaffolds raise ethical concerns towards animal-free procedures. On the other side, synthetic scaffolds offer many benefits, and they are an interesting xenogeneic-free alternative 5, 6 (Table 1).
Moreover, devices such as cell culture inserts can be integrated into 3D cell cultures to facilitate self-assembly and to allow the analysis of microenvironments, soluble factors, interaction between different cells and air-liquid-interface 3D cultures 7, 8, 9, 10, 11.
| Biological scaffolds | Synthetic scaffolds | |
|---|---|---|
| Chemical Composition | Variable | Defined |
| Origin | Animal | Animal-free |
| Mechanical properties | Variability between batches | Defined |
| Reproducibility | Variability between batches | Reproducible results |
| Xenogeneic contaminants | Possible | No |
| Tuneability | No | Yes |
Table 1. Comparison between biologically derived and synthetic scaffolds for 3D cell culture applications 4, 5, 6.
In this application report, we describe the 3D cultivation of human neural stem cells (hNSC) on clear and translucent cellQART® Cell Culture Inserts using PASAP1, a 100% animal free synthetic matrix produced by Nanomed3D. PASAP1 Hydrogel is used to create defined three-dimensional micro-environments for cell culture experiments. PASAP1 nanostructured hydrophilic hydrogel displays multiple functionalizations that fosters attachment, differentiation and viability of a variety of cells. Nanomed3D patented biomaterials are made of synthetic self-assembling peptides. Being 100% synthetic, Nanomed3D scaffolds are pathogen free, pure and reproducible.
Material and Methods
hNSCs cultures
Cells were cultured in untreated flasks (Thermo Scientific) at a density of 104 cells/cm2 using serum-free medium in the presence of basic fibroblast growth factor (bFGF, PeproTech) and EGF (PeproTech) at final concentrations of 10 ng/ml and 20 ng/ml, respectively. Cell cultures were maintained in a humidified incubator at 37°C, 5% O2 and 5% CO2 to allow neurosphere generation. Neurospheres were mechanically dissociated every 10 days in the same growth medium.
3D cell culture
High-density 3D cell cultures of hNSC in PASAP1 (Nanomed3D, Italy) are obtained by a mixing solution containing PASAP1 (1% w/v), 280 mM sucrose solution, 2.5 mM NaOH and hNSCs (4.5 x 104 cells/μl). A droplet (30 μl) of such mixture is placed in the top-center membrane of a clear and translucent 0.4 µm cellQART® Cell Culture Insert (SABEU, GmbH & Co, Northeim, Germany, cat. no. 932 04 12 and 932 04 02) that is placed in a 24-well plate (Thermo Scientific, Nunclon Delta Surface). 700 µL of serum-free neurobasal medium supplemented with bFGF (20 ng/ml, Peprotech) was added to achieve SAP gelation: after 30 minutes of incubation at 37°C, additional 300 µl were slowly added to the top chamber of the insert. At 2 DIV, medium was shifted to a basal medium supplemented with LIF (20 ng/ml, Chemicon) and BDNF (20 ng/ml, Peprotech). In these conditions, the samples were maintained in culture up to 2 weeks.
Immunofluorescence
3D cell culture samples were fixed with 4% paraformaldehyde (PFA), detached from the cell inserts, embedded in OCT and cryosectioned at 100 μm. Slices were washed in PBS. All samples were permeabilized with 0.3% Triton X-100 for 10 minutes at 4°C and treated with 10% normal goat serum (NGS, GIBCO) for 1 hour at room temperature. The following primary antibodies were used: mouse antiβIII-Tubulin (βIII-TUB) (1:500, Biolegend), rabbit anti-glial fibrillary acidic protein (GFAP) (1:500, DAKO), mouse anti-galactocerebroside (GalC) (1:200,Millipore), mouse anti-oligodendrocytes marker O4 (1:200, Millipore). To reveal primary antibodies, the following secondary antibodies were used: goat anti-rabbit Cy3 (1:1000, Jackson), goat anti-mouse Cy3 (1:1000, Jackson), goat anti-rabbit Alexa 488 (1:1000, Invitrogen) and goat anti-mouse Alexa 488 (1:1000, Invitrogen). Cell nuclei were counterstained with DAPI (Molecular Probes). Multiple randomly chosen fields of a minimum of three independent experiments, per each marker and experimental setup, were acquired at 20x magnification via Zeiss Microscope with Apotome System. Quantitative analyses were performed by counting manually positive cells for each marker using NIH-ImageJ software.
Results and Discussion
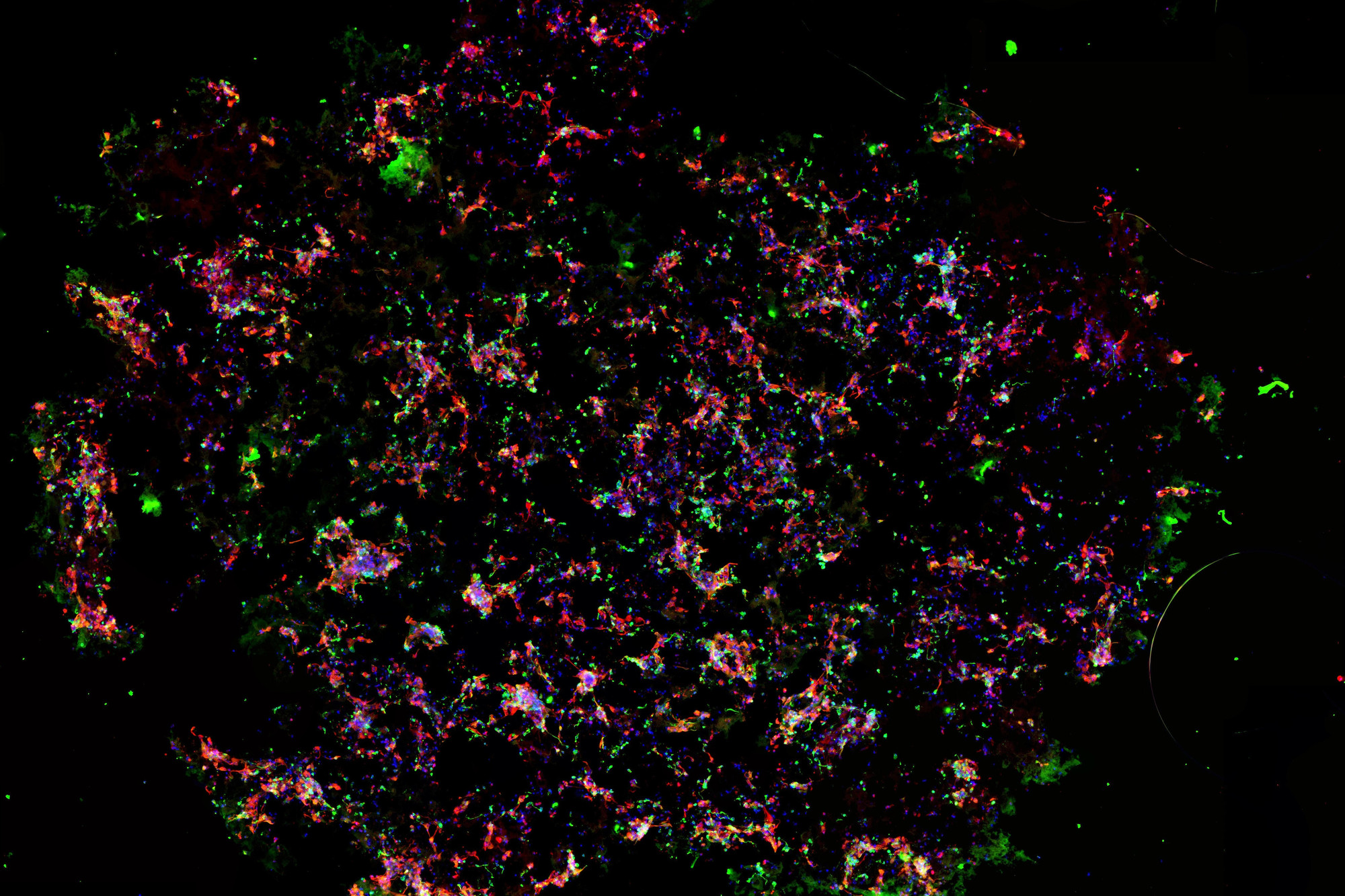
Successful cellular proliferation and differentiation are key elements in hNSC-derived 3D cell cultures. Therefore, we investigated the potential of PASAP1 hydrogel to support survival, growth and neural differentiation on cellQART® Inserts, using immunofluorescence and microscopy studies.
As demonstrated in Figure 2, hNSC embedded in PASAP1 hydrogel attached, grew and colonized the scaffold within 2 weeks in culture. hNSCs were able to proliferate, as indicated by the increased cell density. In addition, cells formed network-like structures and established connections with proximate cells.
Human neural stem cells are multipotent adult stem cells with the ability to differentiate into neurons, oligodendrocytes and astrocytes. The potential of hNSC to generate the main nervous system phenotype in PASAP1 3D cultures was evaluated. As observed in Figure 2 and Figure 3, positive staining for the beta III isoform of tubulin (β-Tub) and glial fibrillary acidic protein (GFAP) were detected. β-Tub is a neuron-specific class III beta-tubulin that is expressed in neurons. Whereas GFAP is an intermediate filament expressed in astrocytes.
To develop a hNSC 3D culture model, cells were embedded in Nanomed3D’s PASAP1 self-assembling peptide hydrogel and cultured on clear and translucent cellQART® Cell Culture Inserts with a pore size of 0.4 µm. PASAP1 provides the typical nanostructured topography of the extracellular matrix, thus mimicking the physiological microenvironment surrounding cells found in living tissues (Figure 1).
A
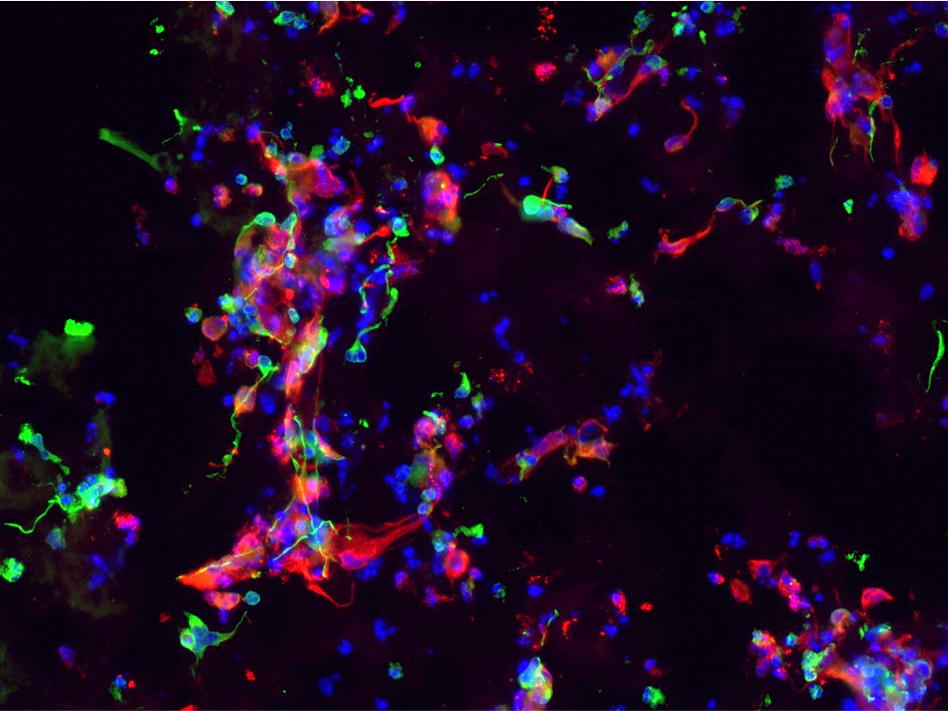
B
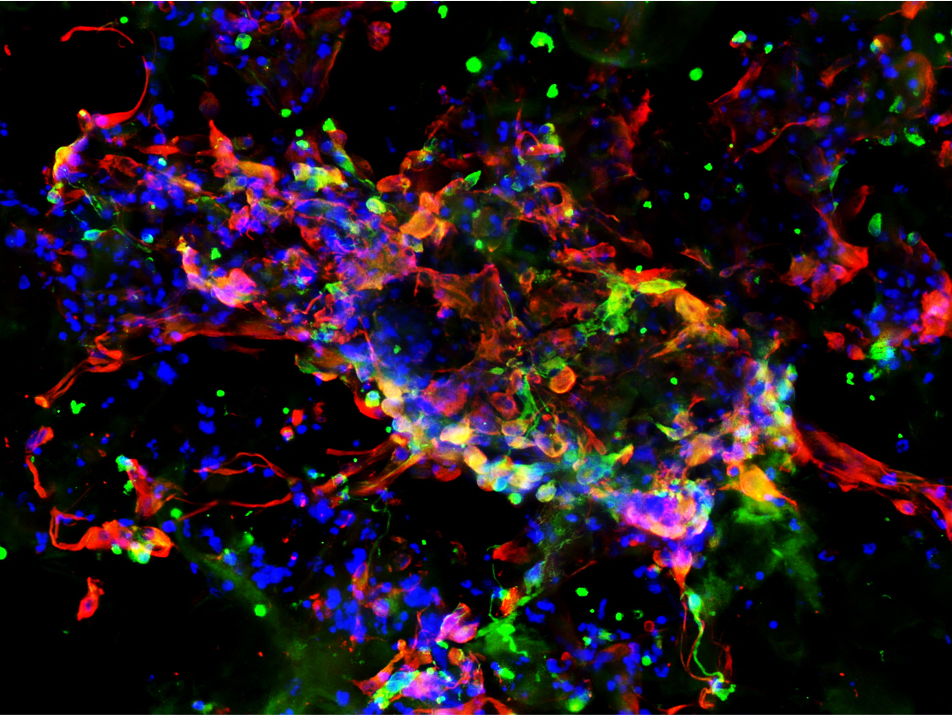
Furthermore, hNSC stained positive for the oligodendrocyte marker GalC-O4 when cultured in PASAP1 3D-cultures for 2 weeks (Figure 4). Oligodendrocytes are cells in the central nervous system that are responsible for the maintenance and generation of the axonal myelin sheath.
A
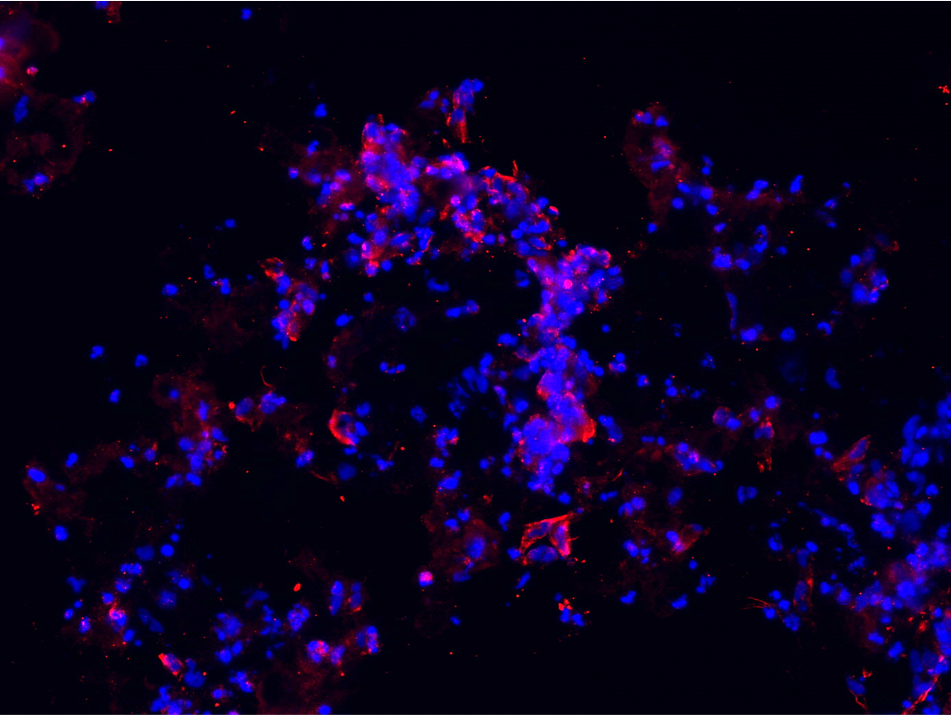
B
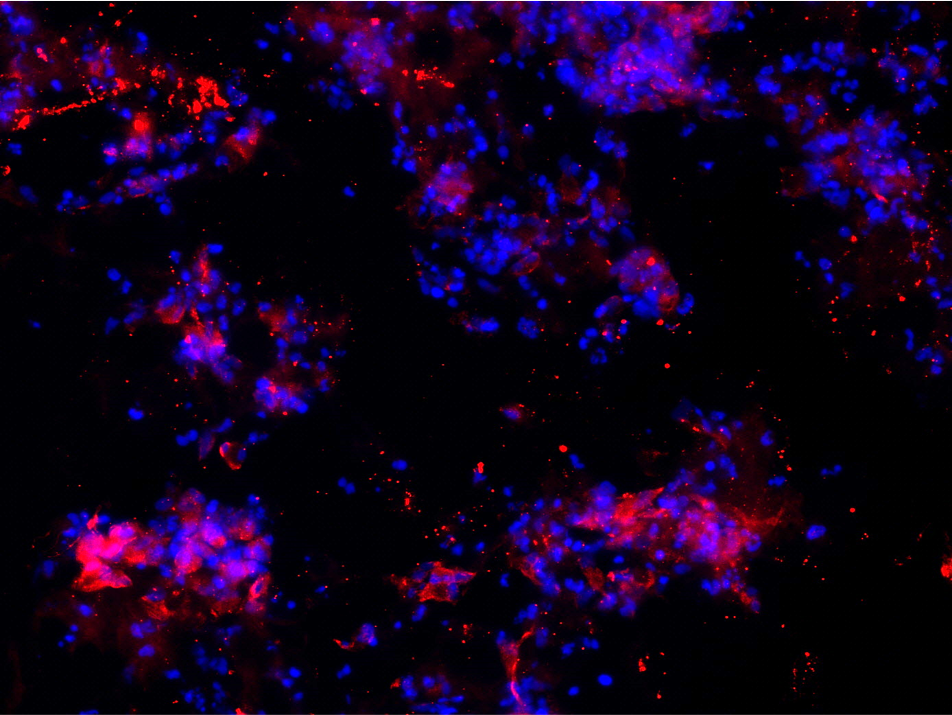
The staining patterns and quantification (Figure 5) were consistent and revealed adequate differentiation of hNSCs when cultured on clear and translucent cellQART® Inserts. No significant difference between the 3D cultures grown on both types of inserts was observed. Interestingly, we observed that the use of cell culture inserts provided a better control on the 3D self-assembly process, increasing reproducibility and minimizing variation. Membrane parameter consistency is of fundamental importance when selecting a cell culture insert to ensure consistent, accurate and reproducible results. The TRAKETCH® Membrane in cellQART® Cell Culture Inserts undergoes a 100% in-line quality control of every segment.
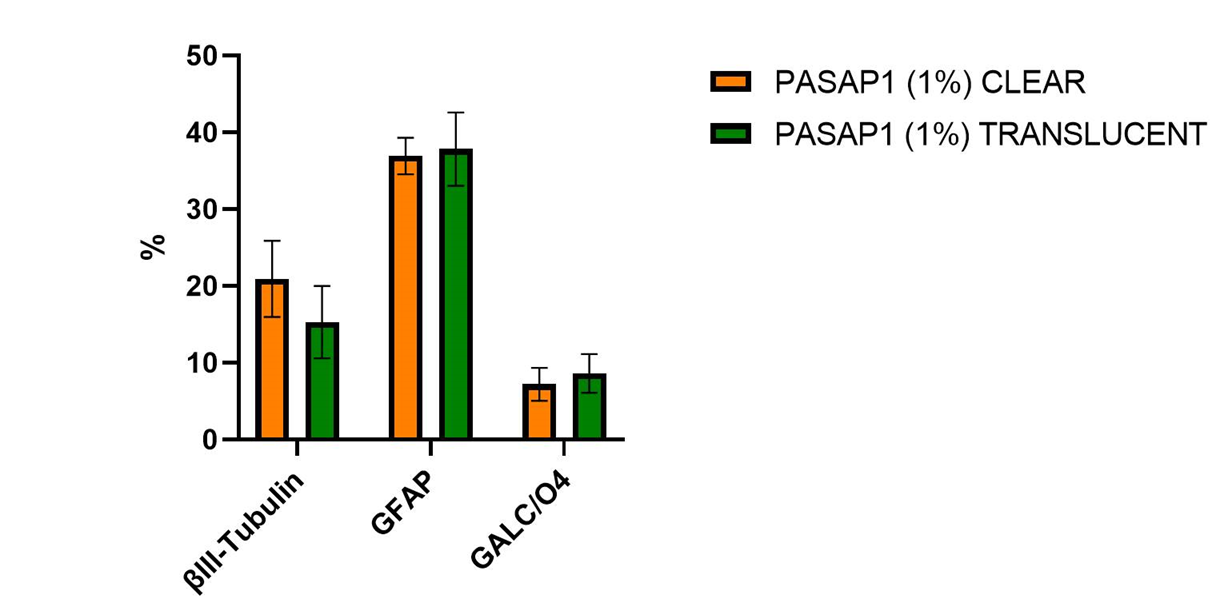
Conclusions
PASAP1 hydrogel provided a viable 3D environment for hNSC growth and neuronal differentiation. The use of cellQART® Cell Culture Inserts contributed to reproducibility by minimizing the variation in self-organised 3D tissue models.
- cellQART® Cell Culture Inserts support the self-assembly, proliferation and differentiation of hydrogel embedded hNSC 3D cultures.
- cellQART® Cell Culture Inserts can be successfully used in combination with PASAP1 hydrogel to generate 3D hNSC-derived cell cultures.
-
PASAP1 hydrogel is a 100% synthetic scaffold that can be easily assembled on cellQART® Cell Culture Inserts for 3D cell culture applications.
-
PASAP1 promotes cell attachment, spreading, survival and differentiation of 3D cultures of human Neural Stem Cells.
- No performance difference was observed between cellQART® clear and translucent Inserts for this specific protocol.
About Nanomed3D (nanomed3d.com)
Nanomed3D is a nanobiotechnology company with more than two decades of expertise in neurodegeneration, tissue engineering, nanomedicine and material science. The company is focused on the design and production of proprietary multi-functionalized self-assembly peptides for research, medical devices and regenerative medicine. Nanomed3D facilities are located in Milan (Italy) and in San Giovanni Rotondo (Italy).
About SABEU (sabeu.com)
SABEU is a leading system provider of microporous filter membranes and injection molded components. In these areas SABEU develops products based on customer specifications, manufacture serial products and set own standards with the cellQART® product line — Made in Germany. SABEU is dedicated to resolve the dilemma of customer needs for innovative cell culture products meeting highest standards at affordable prices. SABEU facilities are located in Northeim (Germany) and Radeberg (Germany).
References
- Slanzi, A., Iannoto, G., Rossi, B., Zenaro, E. & Constantin, G. In vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 8, (2020).
- Shou, Y., Liang, F., Xu, S. & Li, X. The Application of Brain Organoids: From Neuronal Development to Neurological Diseases. Front. Cell Dev. Biol. 8, (2020).
- Lee, C.-T., Bendriem, R. M., Wu, W. W. & Shen, R.-F. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 24, 59 (2017).
- Langhans, S. A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 9, (2018).
- Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
- Marchini, A., Favoino, C. & Gelain, F. Multi-Functionalized Self-Assembling Peptides as Reproducible 3D Cell Culture Systems Enabling Differentiation and Survival of Various Human Neural Stem Cell Lines. Front. Neurosci. 14, (2020).
- Altay, G. et al. Self-organized intestinal epithelial monolayers in crypt and villus-like domains show effective barrier function. Sci. Rep. 9, 10140 (2019).
- Sasaki, N. et al. Development of a Scalable Coculture System for Gut Anaerobes and Human Colon Epithelium. Gastroenterology 159, 388-390.e5 (2020).
- Blutt, S. E. et al. Use of human tissue stem cell-derived organoid cultures to model enterohepatic circulation. Am. J. Physiol.-Gastrointest. Liver Physiol. 321, G270–G279 (2021).
- Li, X., Ootani, A. & Kuo, C. An Air-Liquid Interface Culture System for 3D Organoid Culture of Diverse Primary Gastrointestinal Tissues. Methods Mol. Biol. Clifton NJ 1422, 33–40 (2016).
- Giandomenico, S. L. et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22, 669–679 (2019).
