What do you know about … ALI?
Air-Liquid-Interface cultures
Prepared by: Karina Cuanalo-Contreras, PhD on June 9th, 2021
Update July 17, 2025: cellQART® is partnering with VITROCELL® ALI exposure systems – Find out more >
– Lung Model Edition –
Introduction
Whereas animal models to study lung physiology have provided invaluable information on the understanding of certain conditions such as cystic fibrosis and asthma 1, 2, there is a growing demand for more physiologically relevant human in vitro models according to the 3Rs principle of reducing, replacing and refining the involvement of animals in scientific procedures.
For this purpose, the precise in vitro modelling of the complexity of the airway epithelium is crucial. It is of especial interest the use of adequate, reliable and reproducible models that mimic the pseudostratification of the epithelium, as well as the ability of the airway cells to properly differentiate. In this regard, Air-Liquid-Interface cultures (ALI) offer an exceptional option to model and study the respiratory tract.
What is the respiratory epithelium?
The respiratory epithelium is the lining of the airway tract.
The respiratory epithelium is in charge of 3:
- Protecting from pathogens and debris
- Moisten
- Facilitate gas exchange
The respiratory epithelium is:
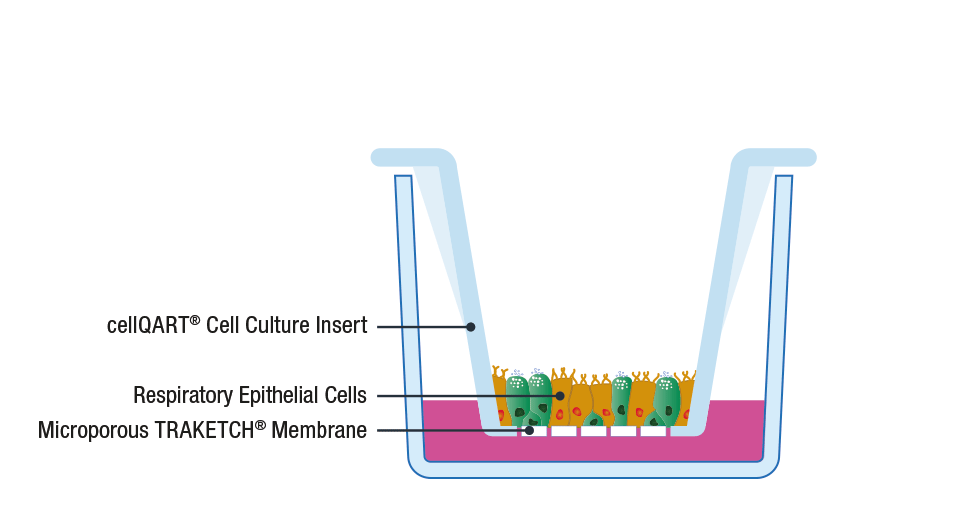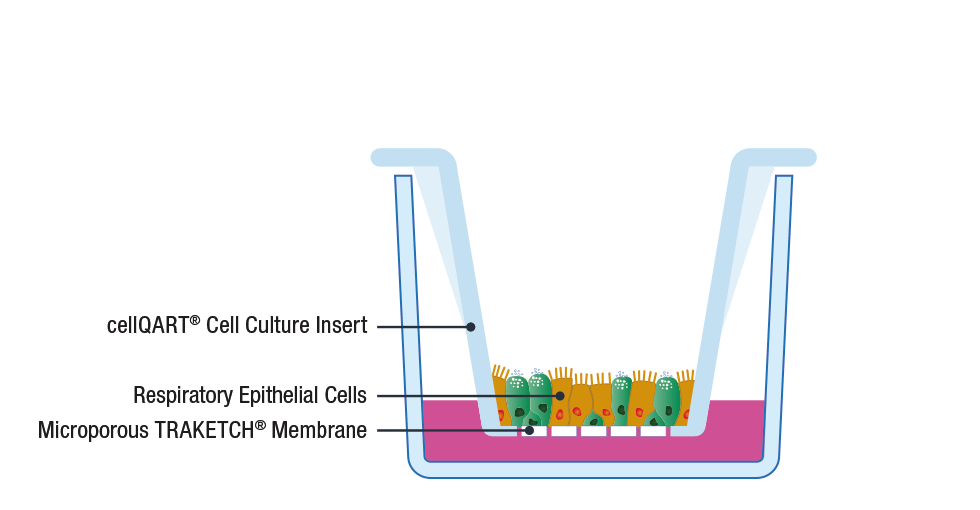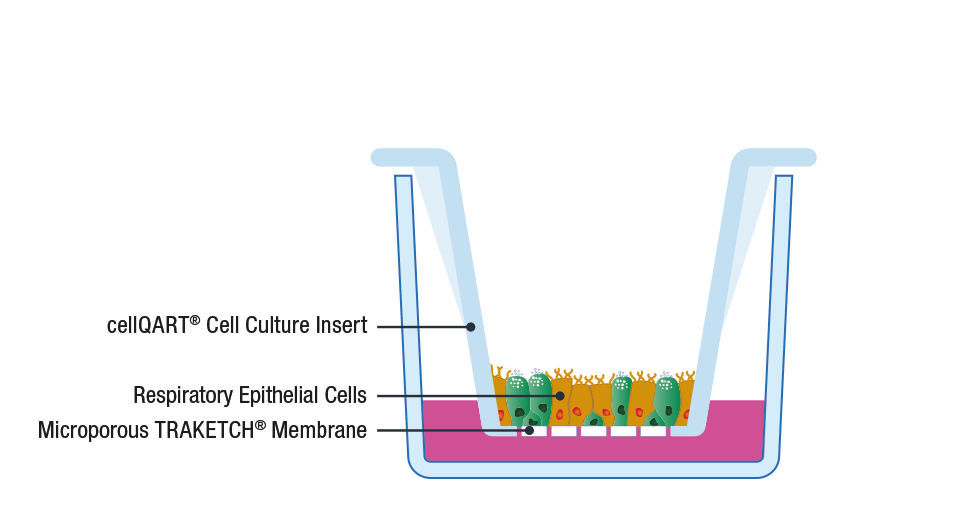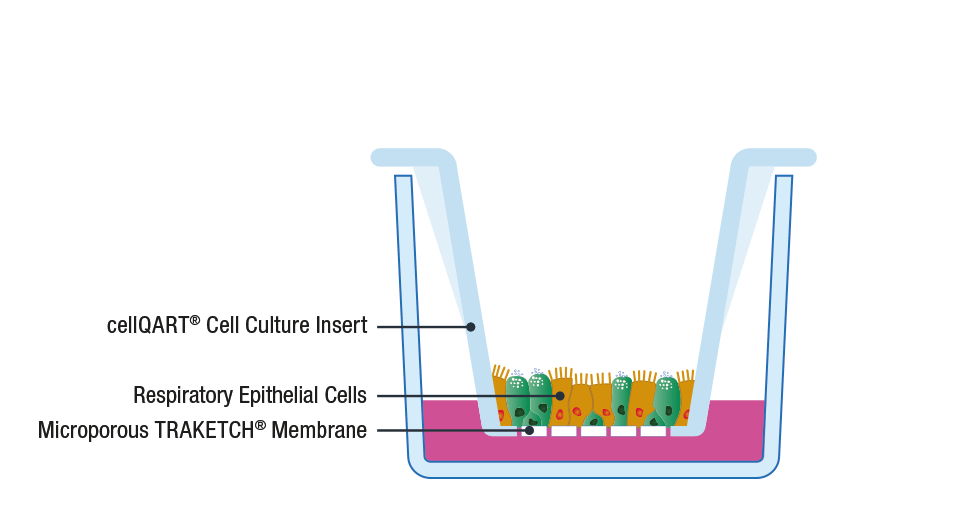
The respiratory epithelium contains the following cell types 6:
- Secretory club cells: produce and secrete mucous
- Ciliated cells: located in the apical side, move mucous across the track
- Basal progenitor cells: bear the capacity to differentiate and restore the epithelial layer as a response to injury
- Rare cell types: tuft cells, solitary neuroendocrine cells, pulmonary ionocytes and goblet cells. There is compelling evidence showing that these cells might be implicated in lung disease 6
Thus, it is of fundamental significance the use of in vitro models that allow the growth and differentiation of common and rare subtypes, as differentiated cells reflect the lung environment in a more reliable way.
What is ALI culture?
In vitro ALI cultures are pivotal and widely used to simulate the respiratory tract.
In lung ALI cultures, airway epithelial cells are grown on permeable membranes at the air-liquid interface. Under this conditions the cells differentiate, polarise and form a pseudostratified epithelium, closely resembling in vivo conditions 6, 7. Airway cells cultivated in this way predict more accurately the normal biology and physiology than cells in submerged culture, where they fail to display the essential mucociliary phenotype.
How to do an ALI culture?
The general procedure to set up an ALI culture starts with the seeding of epithelial cells on a porous support, such as SABEU’s tissue cultured treated TRAKETCH® Membranes of cellQART® Inserts. The typically used inserts/membranes have a pore size of 0.4 µm. The high porosity-translucent membrane is ideal for fast barrier formation, whereas the low porosity-clear membrane has superior optical properties in phase contrast microscopy.
Following cell propagation and confluency achievement, the culture medium from the apical compartment is discarded. By doing this, the apical side of the cells is exposed to the air while only the basal side is supplied with nutrients through the lower compartment. By providing an in vivo-like environment, cells differentiate and polarize, creating a physiological relevant in vitro model 8.
Detailed experimental protocols to perform ALI cultures can be found in the following references 9, 10.
What are some applications of ALI cultures?
- Test aerosolized drugs 11
- Measure the health effect of certain air pollutants 12
- Tobacco research 13, 14
- Perform studies on both normal and disease states 15
- Study of respiratory viral infections e.g. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) 16
The in vitro study of the respiratory system is of cardinal importance to increase our knowledge on the basic mechanisms in normal and altered states. The investigation on the different respiratory epithelium cell subtypes will contribute to underscore the role of each subpopulation in disease and normal airway-surface regulation. This will accelerate the development of potent and targeted therapeutic approaches.
It is important to mention that ALI cultures have many other uses such as in vitro skin models, organoids and biofilm assays. Stay tuned for many more interesting ALI applications.
Have you cited cellQART® in your research paper? Please let us know, we would love to read your paper and learn from your research!
References
- Rosen, B. H. et al.: Animal and model systems for studying cystic fibrosis. J Cyst Fibros 17, S28–S34 (2018)
- Aun, M. V., Bonamichi-Santos, R., Arantes-Costa, F. M., Kalil, J. & Giavina-Bianchi, P.: Animal models of asthma: utility and limitations. J Asthma Allergy 10, 293–301 (2017).
- Kia’i, N. & Bajaj, T.: Histology, Respiratory Epithelium. in StatPearls (StatPearls Publishing, 2020).
- Respiratory cilia: MedlinePlus Medical Encyclopedia Image, https://medlineplus.gov/ency/imagepages/19533.htm.
- Lee, J. L. & Streuli, C. H.: Integrins and epithelial cell polarity. J Cell Sci 127, 3217–3225 (2014).
- Montoro, D. T. et al.: A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324 (2018).
- Ghio, A. J. et al.: Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part Fibre Toxicol 10, 25 (2013).
- Chen, S. & Schoen, J.: Air-liquid interface cell culture: From airway epithelium to the female reproductive tract. Reproduction in Domestic Animals 54, 38–45 (2019).
- Tsoutsoulopoulos, A. et al.: Assessment of the Acute Inhalation Toxicity of Airborne Particles by Exposing Cultivated Human Lung Cells at the Air-Liquid Interface. J Vis Exp (2020) doi:10.3791/60572.
- InSCREENeX: cellQART®. https://cellqart.com/academy/application-reports/air-liquid-interface-ali.
- Lenz, A.-G. et al.: Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions. Am J Respir Cell Mol Biol 51, 526–535 (2014).
- Wang, R. et al.: Complex to simple: In vitro exposure of particulate matter simulated at the air-liquid interface discloses the health impacts of major air pollutants. Chemosphere 223, 263–274 (2019).
- Azzopardi, D. et al.: Evaluation of an air–liquid interface cell culture model for studies on the inflammatory and cytotoxic responses to tobacco smoke aerosols. Toxicology in Vitro 29, 1720–1728 (2015).
- Behrsing, H. et al.: In Vitro Exposure Systems and Dosimetry Assessment Tools for Inhaled Tobacco Products: Workshop Proceedings, Conclusions and Paths Forward for In Vitro Model Use. Altern Lab Anim 45, 117–158 (2017).
- Schögler, A. et al.: Characterization of pediatric cystic fibrosis airway epithelial cell cultures at the air-liquid interface obtained by non-invasive nasal cytology brush sampling. Respiratory Research 18, 215 (2017).
- Mulay, A. et al.: SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell Reports 35, 109055 (2021).
