What do you know about … TEER?
Transepithelial-Transendothelial Electrical Resistance
Prepared by: Karina Cuanalo-Contreras, PhD on April 8th, 2021
Introduction
Tissue barriers are of fundamental importance and play a primary role in homeostasis. They are protective and serve functions such as filtration, secretion, absorption and excretion. Tissue barriers are widespread throughout the body and are composed by epithelial and endothelial layers. The epithelial layer covers organs and cavities, separating them from their surroundings. The endothelial layer comprises the inner vasculature lining and acts as barrier between blood and tissues. Both the epithelial and endothelial layers contain tight and adherent junctions. Tight junctions modulate the flow of ions, solutes, and cells through the intercellular space; whereas adherent junctions control the interplay between cells 1, 2.
In vitro cellular models to study epithelial and endothelial layers are pivotal to elucidate mechanisms of barrier function, drug transport and to understand diseases where barrier tissues are compromised or affected by infections, injuries or drugs. One of the key in vitro techniques to evaluate barrier function is the Transepithelial-Transendothelial Electrical Resistance (TEER assay).
What is TEER assay?
TEER stands for Transepithelial-Transendothelial Electrical Resistance. TEER is a validated, label-free and fast technique to measure the electrical resistance of a barrier tissue model, it is used to monitor live cells at various experimental stages. The electrical resistance is defined as the opposition to current flow in a circuit, it is therefore a quantitative parameter to evaluate integrity of a monolayer through its ionic conductance. It is important to mention that TEER values are in direct proportion to barrier integrity. For example, TEER rises as cells proliferate and it reaches the highest value at confluency, suggesting intactness of the cell layer. On the other side, a reduced TEER value is an indicator of a compromised barrier or loosening of the tight junctions 3, 4.
How is TEER assay performed?
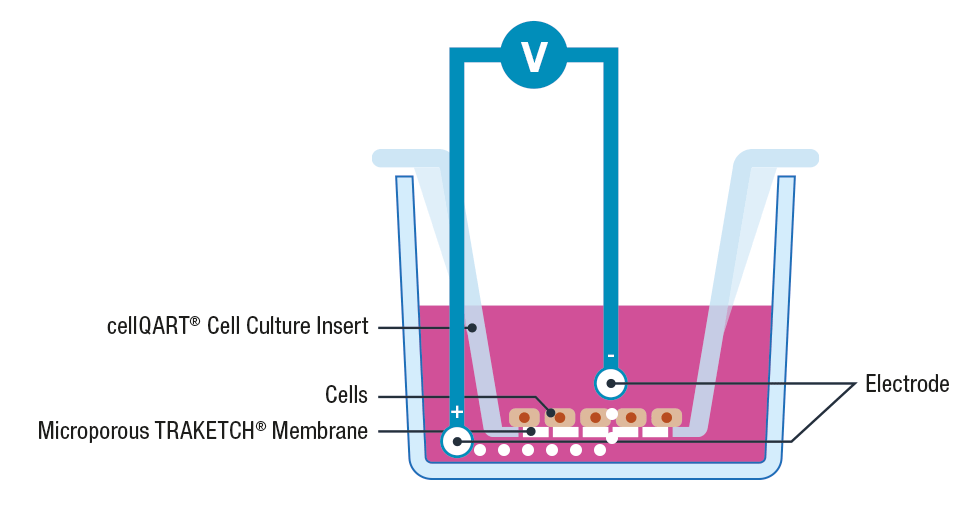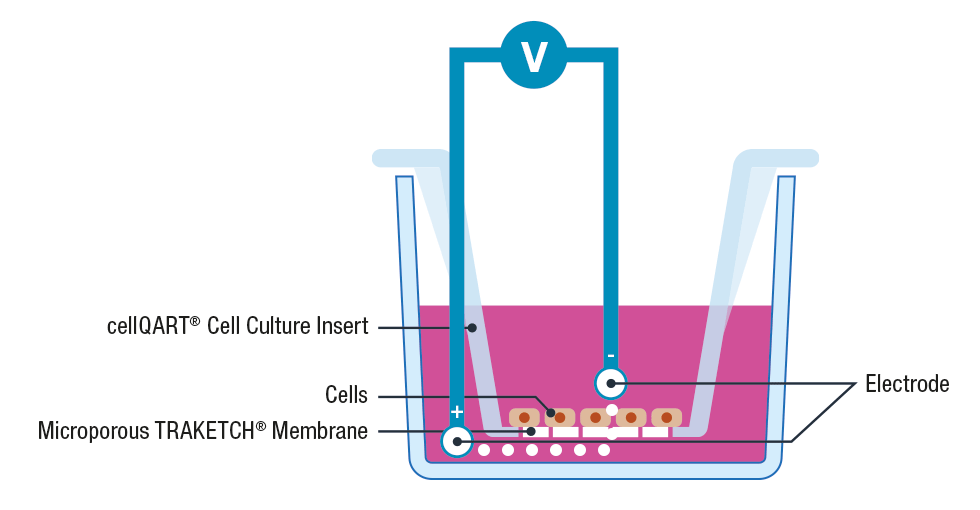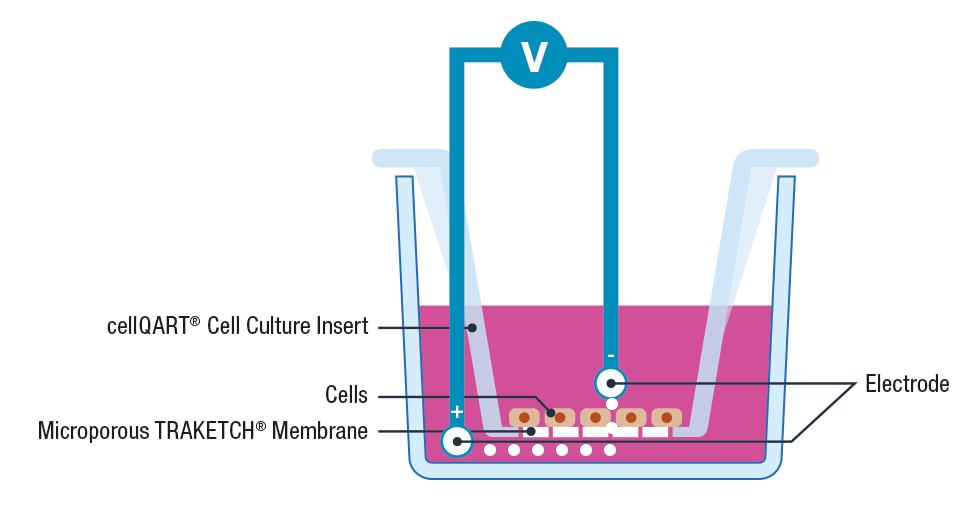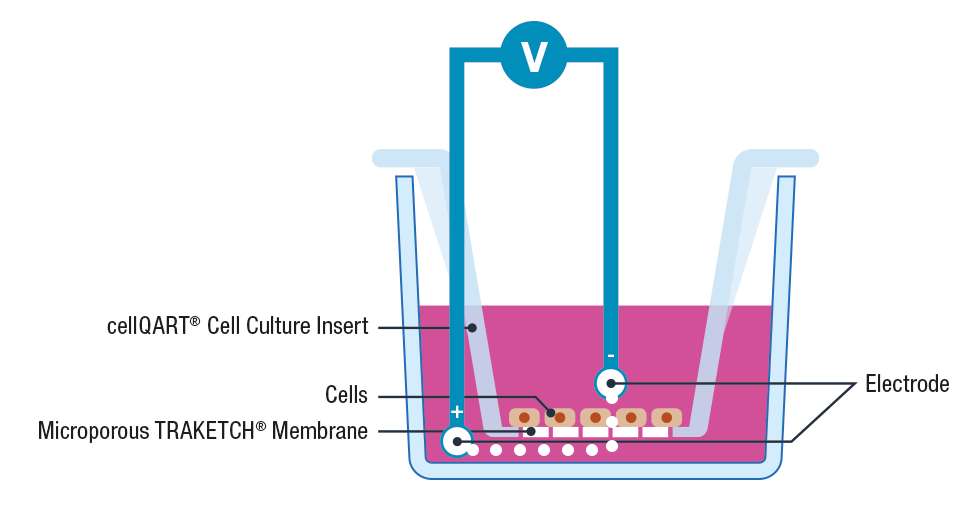
The use of cell inserts is a gold standard to perform TEER assays. Cell inserts contain porous supports and are specially designed to allow the separation into two compartments, simulating the physiological condition where the cell monolayer acts as a diffusion barrier between the apical and basolateral sides.
Briefly, cells are cultured on inserts containing membranes that are semipermeable to ions and cell culture media. Once the cellular monolayer has been established, a pair of electrodes are inserted in both the lower and upper compartments, an A.C. voltage signal is then applied and the resulting resistance is recorded using a Voltohmeter 3, 4.
Please note that to avoid cross contamination between samples it is extremely important to thoroughly clean the electrodes before each measurement. This can be performed by placing the electrodes for 5 minutes in 70% ethanol, allow them to dry and wash any residue with sterile media or buffer.
How is TEER calculated?
The electrical resistance, also known as ohmic resistance, is measured in ohms (Ω). The electrical resistance is in inverse proportion to the area of the cell insert membrane. For this reason, TEER is usually expressed in Ω × cm2, as it takes into account the surface of the membrane (in cm2) 5. For reference, Table 1 contains a useful guide of cellQART® surface membrane growth area.
| Insert type | Surface area | More information |
|---|---|---|
| 6-Well | 4.5 cm2 | Products > |
| 12-Well | 1.1 cm2 | Products > |
| 24-Well | 0.3 cm2 | Products > |
Table 1: Surface area of cellQART® TRAKETCH® membranes in cm2.
The total ohmic resistance is a sum of the resistance of the cell monolayer (net resistance), the resistance of the culture media (Rm), the resistance of the semipermeable membrane (Ri) and the resistance of the interface between the electrodes and the medium (Re). To obtain the net resistance, it is therefore necessary to subtract the total ohmic resistance from the electrical resistance of a blank (cell insert in experimental culture media without cells). Finally, to calculate TEER, the net resistance is multiplied by the membrane area 4, 6.
- Total ohmic resistance (Ω) = Net resistance + Rm + Ri + Re
- Blank resistance (Ω) = Rm + Ri + Re
- Net resistance (Ω) = Total ohmic resistance (Ω) – Blank resistance (Ω)
- TEER (Ω × cm2) = Net resistance (Ω) × Area of the membrane (cm2)
Which parameters affect TEER measurement?
- Temperature:
TEER is inversely proportional to temperature. TEER increases at lower temperatures and vice versa. It is crucial to perform TEER measurements always at the same temperature and to avoid leaving the plates for long periods outside the incubator. It is recommended to perform TEER measurements at 37°C 3, 5. - Electrode positioning:
Both electrodes must be positioned perpendicularly to the bottom of the plate and totally covered by media 3. The lower chamber electrode must touch the bottom of the well and the upper chamber electrode must not touch the membrane. - Membrane quality:
Membrane parameter consistency is of fundamental importance when selecting a permeable support to model tissue barriers. The TRAKETCH® Membrane in cellQART® Cell Culture Inserts undergoes a 100% in-line quality control of every segment and is thus the preferred membrane to ensure consistent, accurate and reproducible results of TEER measurements. - Vibrations:
Vibrations can alter electrode placement and they cause disturbance of the electric field across the monolayer 7.
What are some applications of TEER?
Evaluation of cell monolayer of tissue barriers models:
- Gastrointestinal tract
- Blood-brain barrier
- Airways
- Skin
Study of cell monolayer integrity of disease models where tissue barriers are compromised:
- Cystic fibrosis
- Inflammatory bowel disease
- Viral infections
- Cancer
Pharmacological research:
- Study of the transport of drugs across barrier tissues
- Assessment of integrity of tissue barriers before, during and after drug absorption
- Evaluation of drug toxicity
Have you cited cellQART® in your research paper? Please let us know, we would love to read your paper and learn from your research!
References
- Anderson, J. M. & Van Itallie, C. M.: Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1, a002584 (2009)
- García-Ponce, A., Chánez Paredes, S., Castro Ochoa, K. F. & Schnoor, M.: Regulation of endothelial and epithelial barrier functions by peptide hormones of the adrenomedullin family. Tissue Barriers 4, e1228439 (2016).
- Srinivasan, B. et al.: TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20, 107–126 (2015)
- Elbrecht, D. H. & Hickman, C. J. L. and J. J.: Transepithelial/endothelial Electrical Resistance (TEER) theory and applications for microfluidic body-on-a-chip devices. Journal of Rare Diseases Research & Treatment 1, (2016).
- Urban, F. et al.: P E TER-assay: Combined Impedimetric Detection of Permeability (P E) and Resistance (TER) of Barrier-Forming Cell Layers. Scientific Reports 10, 7373 (2020).
- Benson, K., Cramer, S. & Galla, H.-J.: Impedance-based cell monitoring: barrier properties and beyond. Fluids and Barriers of the CNS 10, 5 (2013).
- Poenar, D. P., Yang, G., Wan, W. K. & Feng, S.: Low-Cost Method and Biochip for Measuring the Trans-Epithelial Electrical Resistance (TEER) of Esophageal Epithelium. Materials (Basel) 13, (2020).
